VIP article “Reversible Dissociation of a Dialumene” from the groups of Dr Mike Cowley (UoE) and Dr Tobias Krämer (MU) in Angewandte Chemie International Edition
Dialumenes are neutral Al(I) compounds that feature Al=Al multiple bonds. Besides their intriguing bonding properties, such low-valent main group compounds hold great promise in bond-activation catalytic transformations. Until recently only two examples of stable dialumenes had been reported. The Cowley group has now successfully isolated and characterised a new amidophosphine-supported dialumene, which was obtained from reduction of a Al(II) diiododialane with Na/K alloy. The X-ray crystallographic structure reveals a long and extreme trans-bent Al=Al bond, diverging substantially from the previous examples. The low dissociation energy and bond-order facilitate dissociation of the dialumenes into monomeric Al(I) species in solution - the first such direct observation in this type of chemistry. The presence of this equilibrium has implications for the reactivity of the compound towards alkenes and alkynes: it can either react as dimer or monomer.
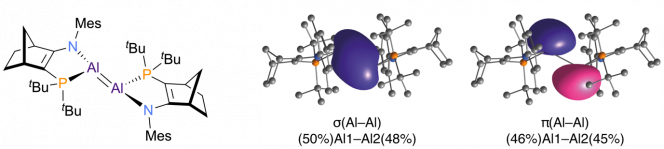
To gain deeper insight into the bonding of this species, a comprehensive quantum chemical analysis of the structure was performed by Keelan Byrne, currently working on a PhD under guidance from Tobias Krämer. It was demonstrated that the charge density of the “pi-bond” is shifted towards the Al centres as a consequence of the ligand properties. In fact, the double bond character is strongly reduced. Intriguingly, the Al centres can invert through a previously unknown flip mechanism, which exchanges the chemical environments of the donor atoms (as probed by 31P NMR spectroscopy). In summary, this study provides detailed insight into the electronic structure and chemistry of low-valent Al compounds and establishes the first guidelines for how bonding and structural features of the Al=Al bond can be systematically controlled by substituent variation.
The results of this study carried out by the groups of Dr Cowley (University of Edinburgh) and Dr Krämer (Maynooth University) have been reported as a joint experimental/theoretical article in Angewandte Chemie International Edition.
(https://onlinelibrary.wiley.com/doi/10.1002/anie.202111385). The manuscript has received a VIP designation - less than 5% of manuscripts accepted in this journal receive such a positive evaluation. ChemistryViews.org have also highlighted the work: https://www.chemistryviews.org/details/ezine/11322102/Multiply-Bonded_AlI_Compounds.html
