Article “Stability and C–H Bond Activation Reactions of Palladium(I) and Platinum(I) Metalloradicals: Carbon-to-Metal H-Atom Transfer and an Organometallic Radical Rebound Mechanism” from the groups of Dr Tobias Krämer (Maynooth) and Dr Adrian Chaplin (Warwick) published in Journal of the American Chemical Society
Palladium and platinum complexes are among the most versatile transition metal catalysts employed in a wide range of chemical transformations in contemporary organic synthesis. Specifically, they find utility in the activation of C–H bonds, typically realised through concerted oxidative addition involving diamagnetic redox couples. In contrast, the organometallic chemistry of paramagnetic derivatives of Pd and Pt is much less developed, especially with reference to the advances being made with d9-complexes of nickel.
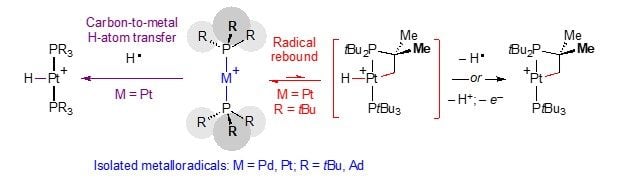
In a collaborative effort, the Chapin and Kraemer groups were now able to demonstrate that C–H bond activation at a platinum(I) bisphosphine complex occurs via a radical rebound mechanism. It was observed that reversible one-electron oxidation of the linear complex Pt(PtBu3)2 generates a transient Pt(I) d9 metalloradical, which undergoes intramolecular cyclometalation of the ligand. The resulting 1:1 mixture of a Pt(II) metallacycle and Pt(II) hydride might suggest net radical oxidative addition of a C(sp3)–H bond across two transient Pt(I) metalloradicals. Insight into the mechanistic details was gained from extensive experimental techniques substantiated by detailed computational exploration oft the reaction landscape. It was shown that the mechanism associated with cyclometallation of [Pt(PtBu3)2]+ follows a monometallic radical rebound mechanism, involving carbon-to-metal hydrogen-atom transfer and formation of an intermediate platinum(III) hydride complex. Such a mechanistic sequence resembles the radical rebound mechanism typical of metal-oxo complexes utilised by Nature.
The results of this study carried out by the groups of Dr Chaplin (Warwick University) and Dr Krämer (Maynooth University), also with involvement of the Macgregor group (Heriot-Watt University), have been reported as an article in J. Am. Chem. Soc. (https://pubs.acs.org/doi/10.1021/jacs.3c04167).
