
Wednesday, September 2, 2020 - 11:45


A new study by the Kathleen Lonsdale Institute for Human Health Research at Maynooth University has unveiled a critical link between the immune system and skeletal muscle wasting. The research has also identified therapeutic targets that may treat inflammatory complications of muscle damage.
The collaborative research was carried out by Dr Paul Dowling of the Lonsdale Institute’s Clinical Proteomics Laboratory; postgraduate student Stephen Gargan and Prof Kay Ohlendieck of the Lonsdale Institute’s Muscle Biology Laboratory.
They worked in close collaboration with Dr Paula Meleady and colleagues at the National Institute for Cellular Biotechnology at Dublin City University, and Prof Dieter Swandulla and co-workers at the Institute of Physiology and the Paediatric Intensive Care unit at the University Hospital of Bonn, Germany.
Muscular dystrophy is a genetic disease that is characterized by the loss of a crucial protein named dystrophin. The lack of dystrophin causes severe damage to muscle cells and eventually results in skeletal muscle weakness, heart disease and serious breathing difficulties in Duchenne muscular dystrophy patients.
This new collaborative study has explored new aspects of the link between chronic inflammation and progressive muscle damage at the biomolecular level.
An interdisciplinary approach used high performance protein screening and bioinformatic modelling of the spleen, in combination with advanced biochemical and cell biological analyses. This approach has revealed a connection, or ‘crosstalk’, between progressive muscle damage and a hyperactive immune system in Duchenne muscular dystrophy.
The findings agree with the concept that morphological changes in lymph nodes are associated with alterations in splenic inflammatory immune cells and their increased migration from the splenic reservoir to injured dystrophic muscle cells.
Based on this newly established interaction between the spleen and muscle damage, new therapeutic targets have been identified that may be useful to treat the inflammatory complications of chronic muscle damage.
The research was published in the prestigious interdisciplinary journal iScience, a new open-access journal from Cell Press, which focuses on applied research that decisively advances a specific field across the life sciences.
The researchers used proteomics in mice suffering from Duchenne muscular dystrophy to model how the skeletal muscles and the spleen influence each other in view of the dystrophin deficiency.
The spleen plays a key role in the immune response and is located in the abdominal cavity near the stomach. It ensures the proliferation of lymphocytes, which are white blood cells, and also stores monocyte-type immune cells and disposes of worn-out red blood cells.
The researchers used the Duchenne mice to decode for the first time the set of proteins (proteome) of the spleen in comparison to healthy control animals and created a comprehensive protein archive for this organ.
Furthermore, the study provides evidence that the loss of the long form of dystrophin, as observed in Duchenne muscular dystrophy in skeletal muscle, apparently causes secondary effects in the lymphatic system. “It’s a real ‘crosstalk’ between skeletal muscles and the lymphatic system,” said lead author Dr Paul Dowling.
“The mice with Duchenne disease showed numerous changes in the proteomic signature of the spleen compared to the controls,” said Prof Kay Ohlendieck.
The term “crosstalk” is used, for example, when there is a disruptive overlay of another conversation on the phone that can be heard in the background. In the specific case of Duchenne muscular dystrophy, the “crosstalk” was particularly expressed by the fact that the short form of the dystrophin was still produced as normal in the spleen, but there were disruptive changes of the proteomic signature in the other protein species.
The researchers point out that the results of the study suggest that the mechanisms of the inflammatory processes which occur in the course of Duchenne muscular dystrophy merit special attention. This is because these inflammatory mechanisms are an important feature of muscle fiber degeneration and contribute significantly to the progression of the disease. “The specific interactions of the dystrophin deficiency with the immune system might therefore open up new therapeutic approaches,” said Prof Swandulla.
The study was funded by Science Foundation Ireland (SFI) and the postgraduate research programme of the Lonsdale Institute which focuses on improving our understanding of fundamental aspects of human health and disease.
Illustration of crosstalk between the spleen and muscle damage in muscular dystrophy
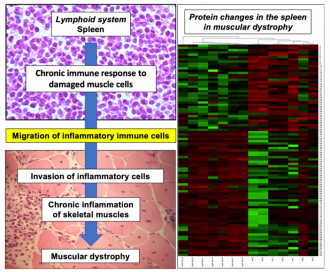

Dowling, P., Gargan, S., Zweyer, M., Henry, M., Meleady, P., Swandulla, D., Ohlendieck, K., Proteome-wide changes in the mdx-4cv spleen due to pathophysiological crosstalk with dystrophin-deficient skeletal muscle, ISCIENCE (2020), in press.
